Scoping a Possible Data Expedition – Big Pharma Payments to Doctors
Do we need a register of interests for medics?
Picking up on an announcement earlier this week by GlaxoSmithKline (GSK) about their intention to “move to end the practice of paying healthcare professionals to speak on its behalf, about its products or disease areas, to audiences who can prescribe or influence prescribing …. [and to] stop providing financial support directly to individual healthcare professionals to attend medical conferences and instead will fund education for healthcare professionals through unsolicited, independent educational grant routes”, medic, popular science writer and lobbiest Dr Ben Goldacre has called for a register of UK doctors’ interests (Let’s see a register of doctors’ interests) into which doctors would have to declare payments and benefits in kind (such as ‘free’ education and training courses) received from medical companies. For as the GSK announcement further describes, “GSK will continue to provide appropriate fees for services to healthcare professionals for GSK sponsored clinical research, advisory activities and market research”.
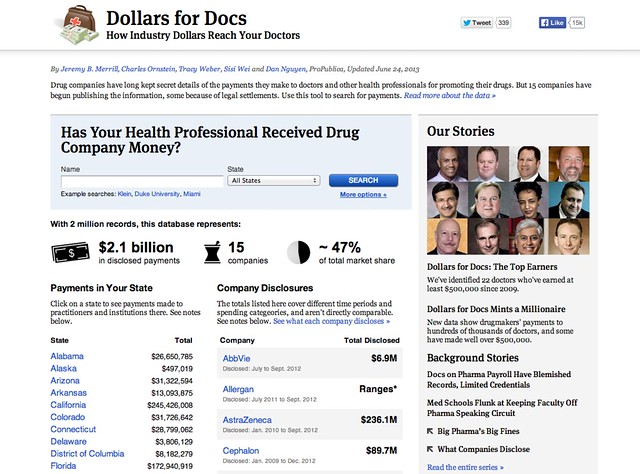
An example of what the public face of such a register might look like can be found at the ProPublica Dollars for Docs site, which details payments made by several US drug companies to US practitioners.
The call is reinforced by the results of a public consultation on a register of payments by the Ethical Standards in Health and Life Sciences Group (ESHLSG) published in October 2013 which showed “strong support in the healthcare community and across life science companies for the public disclosure of payments through a single, searchable database to drive greater transparency in the relationship between health professionals and the companies they work with.”
The call for a register also sits in the context of an announcement earlier this year (April 2013) by the Association of the British Pharmaceutical Industry that described how the pharmaceutical industry was “taking a major step … in its on-going transparency drive by beginning to publish aggregate totals of payments made last year to doctors, nurses and other healthcare professionals.” In particular:
[t]hese figures set out the details of payments made by ABPI member companies [membership list] relating to sponsorship for NHS staff to attend medical education events, support such as training and development, as well as fees for services such as speaking engagements to share good clinical practice and participation in advisory boards. Companies will also publish the number of health professionals they have worked with who have received payments
Payments from pharma into the healthcare delivery network appear to come in three major forms: payments to healthcare professionals for consultancy, participating in trials, etc; medical education payments/grants; payments to patients groups, support networks etc.
(A question that immediately arises is: should any register cover professional service payments as well as medical education payments, for example?)
The transparency releases are regulated according to the The Association of the British Pharmaceutical Industry’s (ABPI) Code of Practice. Note that other associations are available! (For example, the British Generic Manufacturers Association (BGMA).)
A quick look at a couple of pharma websites suggests that payment breakdowns are summary totals by practice (though information such as practice code is not provided – you have to try to work that out from the practice name).
- GlaxoSmithKline transparency minisite (includes links to sites relating to payments to patient groups as well as doctors); example payments to doctors data [PDF];
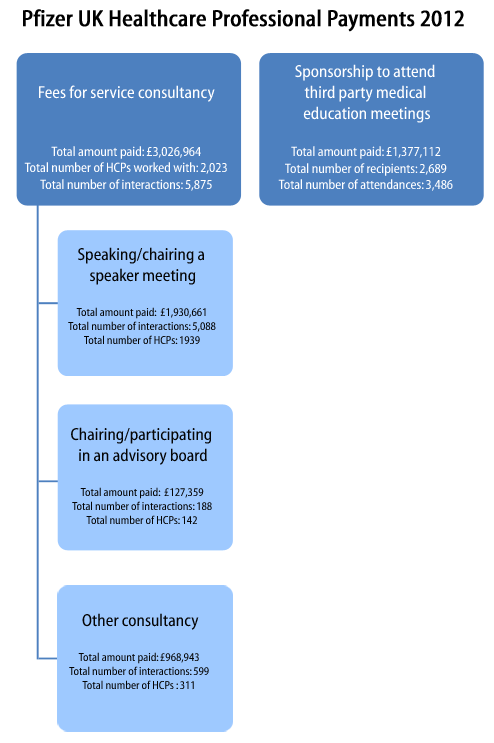
- Pfizer ‘working with healthcare professionals’ minisite (see submenus; example payments data [PDF] (about); site reveals a useful acronym – MEGS: Medical Education Grants;
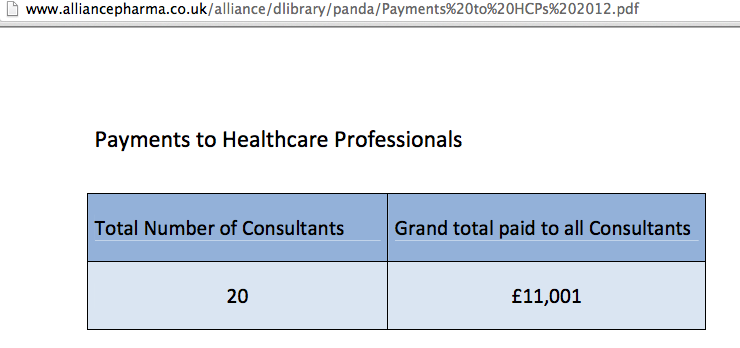
- Alliance Pharma transparency site; example payments data
As the Alliance Pharma transparency report shows, the data released does not need to be very informative at all…
Whilst the creation of a register is one thing, it is likely to be most informative when viewed in the context of a wider supply chain and when related to other datasets. For example:
- clinical trials make use of medical practices in the later stages of a drug trial. To what extent to is participation in a clinical trial complemented by speaking engagements, educational jollies and prescribing behaviour? (Prescribing data at a practice level is available from the HSCIC.);
- regulation/licensing of new products; this is a missing data hook, I think? One of the things that would help to close the loop a little in order to keep tabs on which practices are prescribing from which manufacturers would be a register or datafile that allows you to look up drugs by manufacturer or manufacturer by drug (eg the commercial electronic Medicines Compendium or Haymarket’s MIMs). In the UK, the Medicines and Healthcare Products Regulatory Agency regulates drug manufacture and issues marketing authorisations; but I don’t think there is any #opendata detailing manufacturers and the drugs they are licensed to produce?
- pricing (eg the UK Pharmaceutical Price Regulation Scheme 2014). If we look at prescribing data and find some practices prescribing branded drugs where cheaper and/or generic alternatives are available, is there any relationship with manufacturer payments? That is, can we track the marketing effectiveness of the manufacturers’ educational grants?!
- marketing of medicines to doctors, that is, things like the medical education grants;
- I’m not sure if pharmacists have any discretion in the way they issue drugs that have been prescribed by a doctor. To what extent are medicines marketed to pharmacists by pharma and to what extent do pharmacists which compounds from which manufacturers to buy in and then hand over the counter?
- organisational considerations: many GP practices are part of larger commercial groupings (eg CareUK or Virgin Care). I’m not sure if there is open data anywhere that associates GP practice codes with these wider parent corporate groupings? One question to ask might be the extent to which pharma payments map onto practices that are members of a particular corporate grouping (for example, are there ties up at a strategic level with parent companies?) Associated with this might be investigations that explore possible links with medics who have received payments from pharma and who sit on commissioning groups, and whether prescribing within those commissioning group areas unduly favours treatments from those pharma companies?
Educational payments to doctors by the drug manufacturers may be seen as one of the ways in which large corporations wield power and influence in the delivery and support of public services. In contrast to lobbying ‘at the top’, where companies lobby governments directly (for example, The Open Knowledge Foundation urges the UK Government to stop secret corporate lobbying), payments to practitioners and patient support groups can be seen as an opportunity to engage in a lower level form of grass roots lobbying.
When it comes to calls for disclosure in, and publication of, registers of interests, we should remember that this information sits within a wider context. The major benefit of having such a register may not lay solely in the ability to look up single items in it, but as a result of combing the data with other datasets to see if there are any structural patterns or correlations that jump out that may hint at a more systemic level of influence.